Introduction: Chimeric antigen receptor T-cell therapy (CAR-T) targeting CD19 is a new standard of care for diffuse large B-cell lymphoma (DLBCL) patients with primary refractory disease, early relapse after chemoimmunotherapy, or multiply relapsed disease. Three CD19-directed CAR-T products, axicabtagene ciloleucel (axi-cel), tisagenlecleucel (tisa-cel), and lisocabtagene maraleucel (liso-cel), are FDA approved for relapsed/refractory (R/R) DLBCL and may cure approximately 30-40% of patients. Cytokine release syndrome (CRS), a systemic inflammatory response associated with CAR-T expansion, is a common early toxicity occurring in 42-93% of DLBCL patients in the pivotal phase 2 trials. We hypothesized that development of CRS may be associated with post-CAR-T efficacy outcomes.
Methods: We retrospectively evaluated post-CAR-T outcomes for 57 consecutive patients with R/R DLBCL who received axi-cel or liso-cel infusion at the University of California San Francisco between June 2018 and December 2022. Early post-CAR-T toxicities including CRS and immune effector cell-associated neurotoxicity syndrome (ICANS) were recorded using ASTCT consensus grading. Post-CAR-T efficacy endpoints including overall response rate (ORR), complete remission (CR) rate (best response), progression-free survival (PFS), and overall survival (OS) were evaluated for the entire cohort and compared between patients who developed any grade CRS or no CRS after CAR-T infusion. Of note, patients with high-grade B-cell lymphoma or who received tisa-cel were excluded from this study to eliminate potential confounding given poorer outcomes in these subgroups and imbalance in their relative proportions between the CRS and no CRS cohorts.
Results: For the entire cohort, the median age was 63 (range 26-81) and 59% of patients were male. Diagnoses included de novo DLBCL (65%), transformed follicular lymphoma (30%), and primary mediastinal B-cell lymphoma (5%). Patients had a median of 2.5 systemic therapies (range 1-8) prior to CAR-T infusion. CAR-T products included axi-cel (70%) or liso-cel (30%). 82% of patients received fludarabine and cyclophosphamide lymphodepletion, and the remaining 18% received bendamustine (during the national fludarabine shortage).
Forty-one patients (72%) experienced CRS (grade 1: 35%, grade 2: 37%), and 16 patients (28%) never developed CRS. Seventeen patients (30%) developed ICANS (grade 2: 12%, grade 3: 16%, grade 4: 2%), which occurred exclusively among patients in the CRS cohort. 93% of patients in the CRS cohort received at least one dose of tocilizumab. Baseline characteristics were well balanced between the CRS and no CRS groups with no significant differences in age, sex, performance status, DLBCL subtype, number of prior therapies, CAR-T product administered, lymphodepletion administered, or lactate dehydrogenase at the time of infusion.
For the entire cohort, the ORR and CR rate were 86% and 70%, respectively. With a median follow-up of 1.1 years (range 0.2-4.5 years), the 1-year PFS and OS estimates were 61% and 91%, respectively. Median PFS was 1.7 years and median OS was not reached. Outcomes were similar for patients receiving axi-cel and liso-cel (1-year PFS 62% vs 60%, p=0.94).
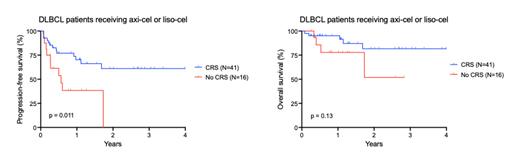
For patients in the CRS cohort vs no CRS cohort, the ORR was 90% vs 75% (p=0.14) and CR rate was 73% vs 63%, respectively (p=0.44). Patients in the CRS cohort had superior PFS compared to patients with no CRS (1-year PFS 70% vs 38%, HR 0.36, 95% CI 0.14-0.98, logrank p=0.011), but there was no significant difference in OS ( Figure 1). There was no significant difference in PFS between patients with grade 1 vs grade 2 CRS (1-year PFS 64% vs 75%, p=0.46).
Conclusions: Although limited in sample size, our study suggests that development of CRS may be associated with more favorable post-CAR-T efficacy including superior PFS compared to patients with no CRS after axi-cel or liso-cel infusion. The more favorable outcomes in patients experiencing CRS may reflect better CAR-T proliferation and cytotoxicity compared to patients with no CRS. These findings suggest a relationship between early post-CAR-T toxicity and efficacy outcomes but require validation in larger datasets.
Disclosures
Spinner:Kite: Consultancy. Andreadis:Gilead: Honoraria; BMS: Honoraria, Research Funding; pharmacyclics: Honoraria; Novartis: Research Funding; Lilly: Research Funding; Roche: Research Funding; Astra Zeneca: Honoraria; Epizyme: Honoraria; Merck: Research Funding. Seshadri:Eli Lilly: Research Funding; Kite: Consultancy; Roche: Research Funding; BeiGene: Consultancy. Ai:Secura Bio: Membership on an entity's Board of Directors or advisory committees; Kyowa Kirin: Honoraria; Biomerieux: Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal